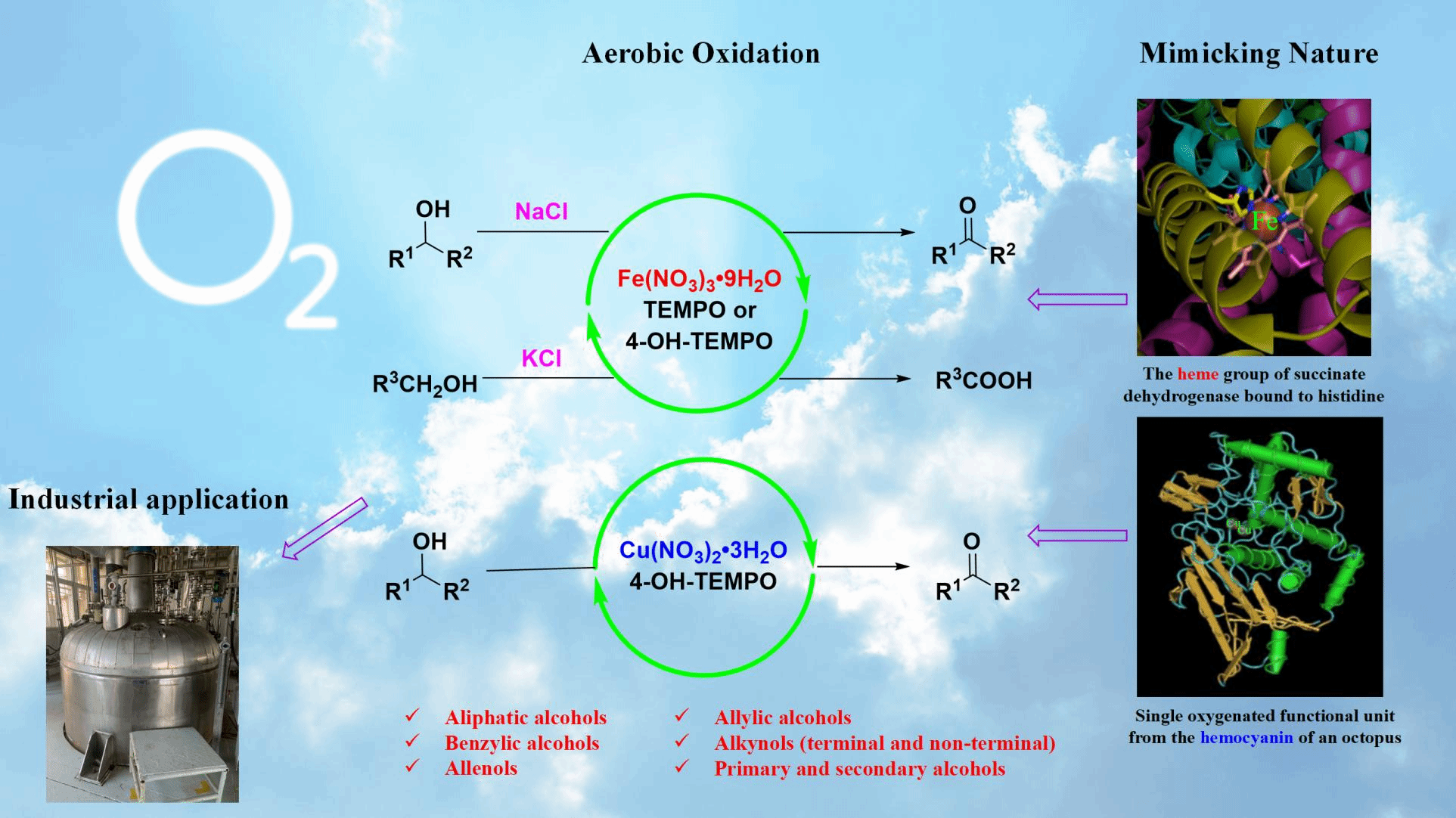
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells (erythrocytes) of almost all vertebrates as well as the tissues of some invertebrates. Hemocyanin containing two copper atoms that reversibly binds a single oxygen molecule is metalloprotein that transport oxygen throughout the bodies of some invertebrate animals, such as helix pomatia and horseshoe crab. Thus, iron and copper are the metals of choice in nature to carry oxygen. Getting inspired by nature, our group has developed a series of methods to realize aerobic oxidation of different types of alcohols including allenols, alkenols, proparglic alcohols, benzylic alcohols, and aliphatic alcohols to afford ketones, aldehydes or acids at room temperature with excellent selectivity. Readily available and cheap Fe(NO3)3·9H2O or Cu(NO3)2·3H2O/TEMPO or 4-OH-TEMPO combinations were used as the catalysts and the counter ion effect of inorganic chloride plays a magical role in our protocol. In particular, our practical and efficient environmentally benign approaches have been employed in industrial production, for example, the spice production.
Applications by other groups
These aerobic oxidation reactions of alcohols have been used by over 20 different research groups in their projects. The data were updated on March 15th 2023 merely based on data-mining via SciFinder. Click the Link for the details.