Abstract
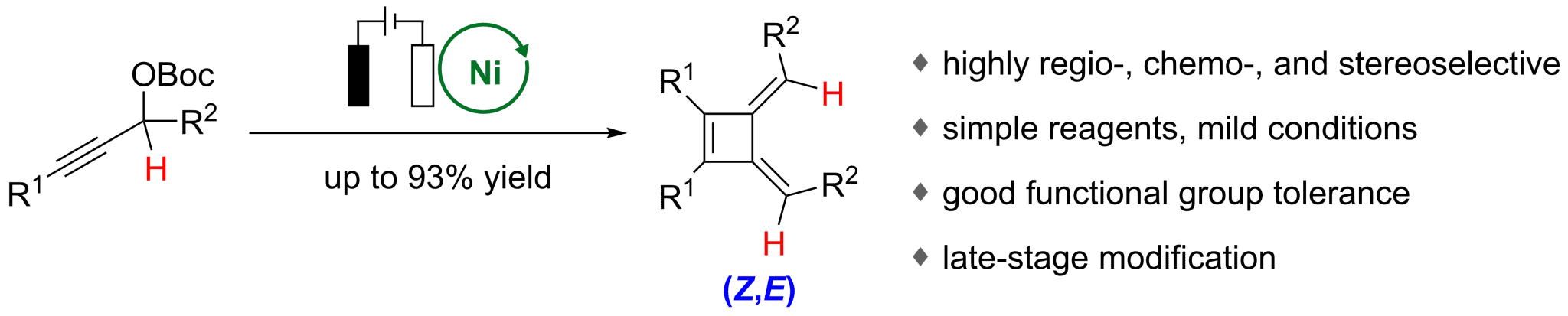
The highly selective construction of highly strained frameworks remains a formidable synthetic challenge. We report here an electrochemical nickel-catalyzed strategy that enables the regio-, chemo-, and stereoselective synthesis of butafulvenes, constitutional isomers of benzene featuring a cyclobutene core and two exocyclic double bonds. This protocol has been achieved via direct electroreductive coupling of propargylic esters under mild conditions with earth abundant metal catalysis of nickel and electricity, affording butafulvenes. Notably, it overcomes the significant E/Z selectivity challenge of secondary propargylic substrates, which previously yielded inseparable mixtures. The success arises from redox-governed sequential generation and transformation of allenyl–Ni, bisallenyl–Ni, bisallene, and five-membered nickelacycle intermediates under electrochemical conditions. Mechanistic studies and cyclic voltammetry support a pathway involving sequential Ni0-involved oxidative addition and reductive coupling as well as Ni0-mediated highly stereoselective cycloisomerization. The reaction exhibits a broad substrate scope accommodating many synthetically versatile functional groups, such as bromide, carboxylate, trifluoromethyl, ether, and silyl groups, and is amenable to late-stage functionalization of complex molecules. This work establishes a general and sustainable platform for accessing strained antiaromatic π-systems and highlights the power of electro-nickel catalysis in stereoselective molecular assembly.
Simiao Zhang (张思淼)