Abstract
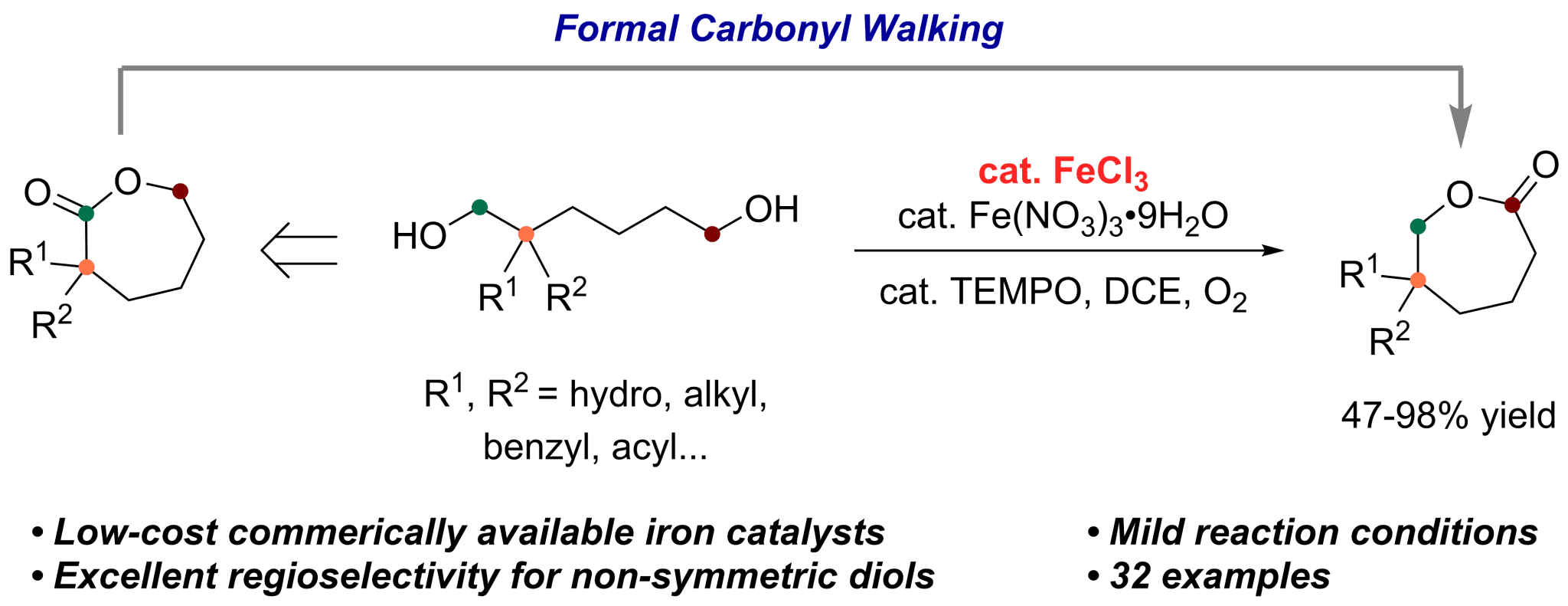
Selectivity control is a very challenging topic in catalytic oxidations of alcohols using oxygen as oxidant. Of particular interest is the catalytic aerobic lactonization of diols, due to the selectivity issue referring to the two hydroxy groups, the reaction afforded a mixture of isomeric products. Herein, we report an efficient catalytic recipe of easily available earth-abundant metal catalysts Fe(NO3)3·9H2O and FeCl3 together with TEMPO for highly selective aerobic oxidative lactonization of 1,6-diols affording a variety of ε-caprolactones. The reaction was conducted under mild conditions with an O2 balloon or under ambient air enjoying high chemo- and regioselectivity for the oxidative lactonization of non-symmetric diols by subtle recognition of the steric environment of the two hydroxy groups. Furthermore, reduction and the selective aerobic oxidative lactonization constitute a formal carbonyl walking over the ε-caprolactones. Mechanistic experiments reveal a process with a hemiacetal as the intermediate. This protocol unlocks an efficient pathway to monomers for polycaprolactones.
Co-first Authors


Sheng Jiang (蒋晟) Yibo Yu (于一博)