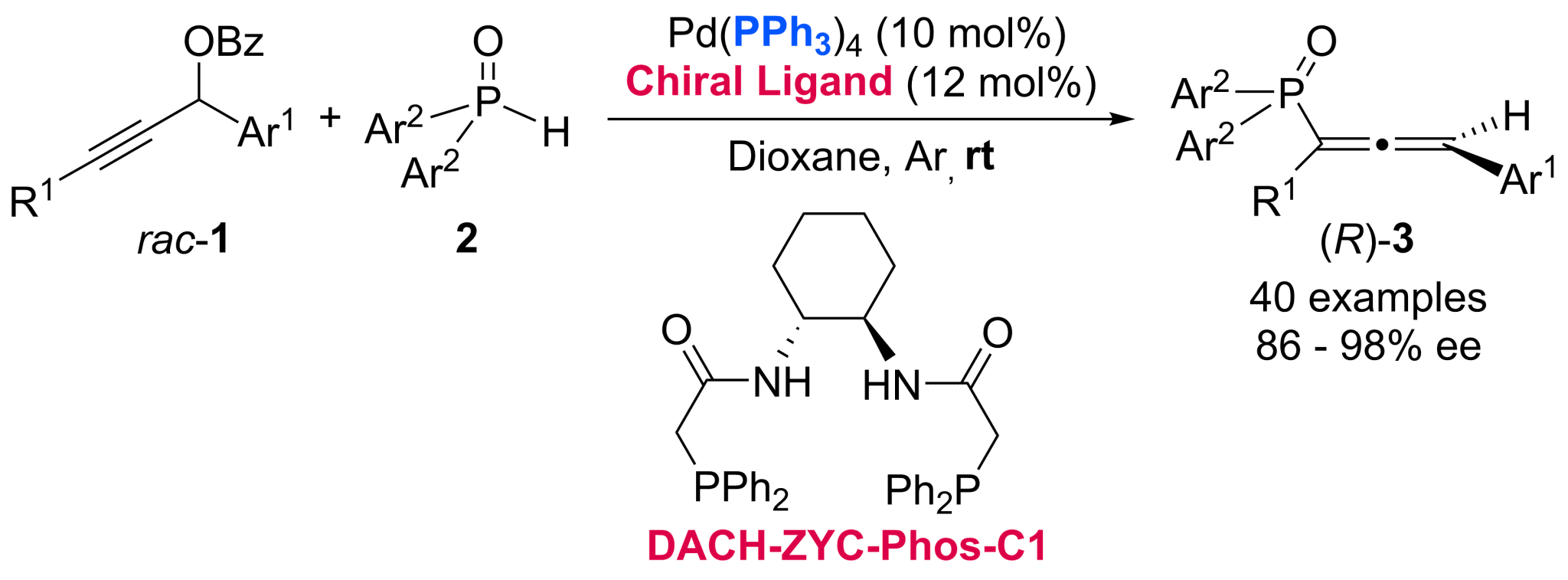
Chiral allenyl phosphine oxides hold significant application value in organic synthesis, serving as both efficient catalysts, ligands, and versatile synthons. However, the development of transition metal-catalyzed asymmetric synthesis for these compounds remains unexplored, primarily due to the facile tautomerization of phosphine oxides into trivalent R1R2P-OH species, which may severely deactivate transition metal catalysts through strong coordination interactions. Herein, a ligand relay strategy has been applied to address this challenge in the Pd-catalyzed enantioselective coupling reactions of propar-gylic benzoates and secondary phosphine oxides (SPOs) to afford chiral trisubstituted allenyl phosphine oxides in high ee. The ligand relay protocol involves initial coordination of a palladium catalyst with readily available triphenylphosphine followed by the dynamic ligand exchange with the stretchable chiral ligand of DACH-ZYC-Phos-C1 (L26) while PPh3 is not working as a non-chiral ligand to any palladium catalysts for this transformation. Chiral trivalent organophosphines with high ee have been obtained upon reduction of allenyl phosphine oxides. Mechanistic studies revealed the nature of kinetic resolution. DFT calculations provide a mechanistic rationale for the observed high enantioselectivity and superior catalytic efficiency, which is governed by the ligand L26 through steric repulsions that selectively destabilize the disfavored oxidative addition transition states.