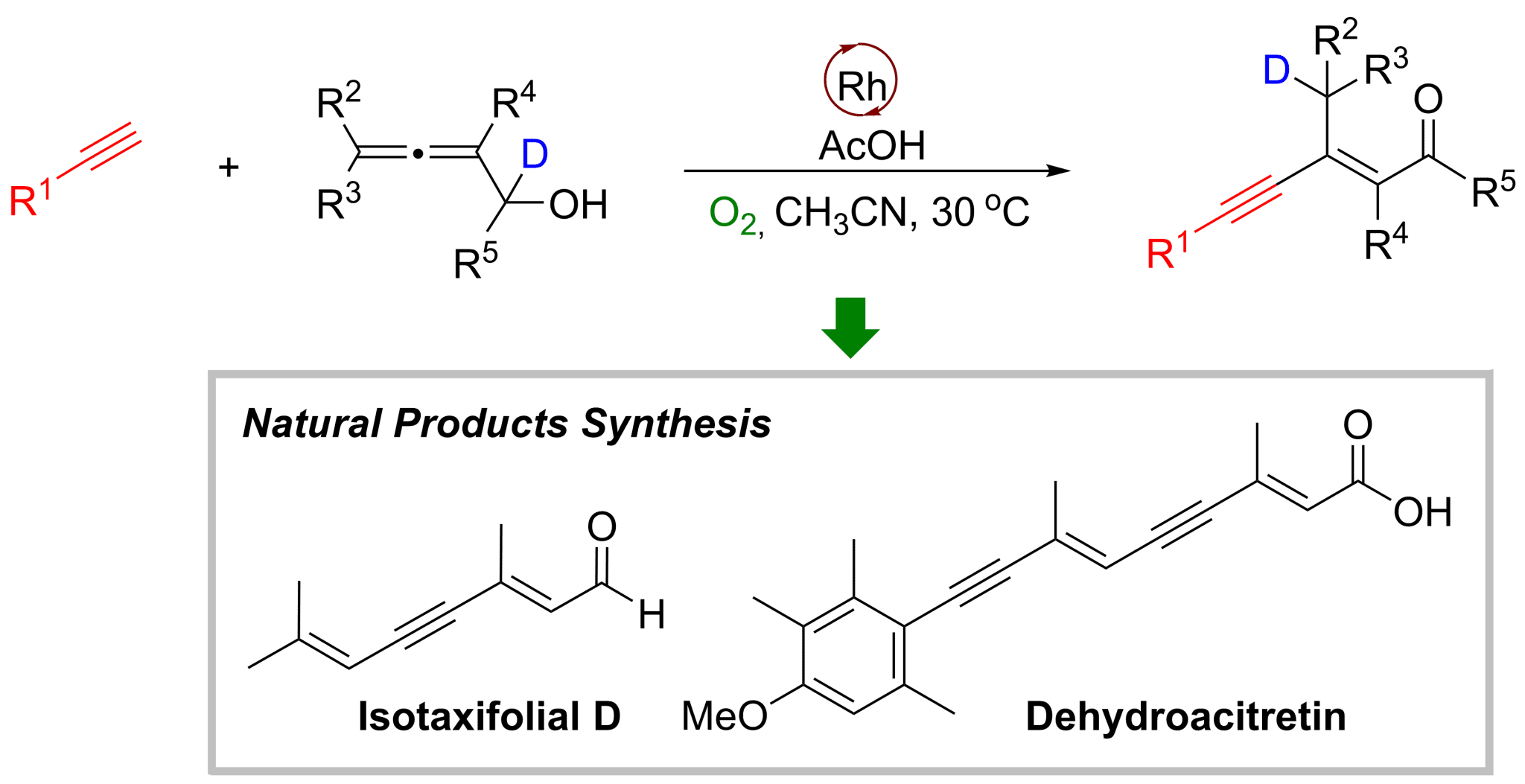
A rhodium-catalyzed highly chemo-, regio-, and stereo-selective linear cross coupling reaction of terminal alkynes with readily available 2,3-allenols afforded the synthetically and biologically versatile 3-alkyn-2(E)-enones/enals. The reaction exhibits a perfect match of the reactivities for these two classes of compounds since the well-known self-reacting products of both reactants were not formed. The reaction enjoys a wide substrate scope under ambient conditions. Furthermore, the synthetic potentials have been demonstrated: by utilizing this reaction as a pivotal step, a very concise two-step total synthesis of naturally occurring isotaxifolial D has been achieved; an efficient synthesis of dehydroacitretin by applying this reaction for two times has also been executed. Through mechanistic studies including D-labelling experiments, it is proposed that the reaction proceeded via terminal alkynylmetalation, stereodefined insertion providing 2-alkynyl p-allylic intermediate, which would undergo C-C single bond rotation, intramolecular ligand exchange, b-H elimination, and reductive elimination resulting in 1,4-H delivery to afford the final enynes. The reaction circumvents the use of a stoichiometric amount of base under the catalysis of a single Rh catalyst since terminal alkynylmetalation is base free and the catalyst is regenerated by aerobic oxidation in the presence of proton.