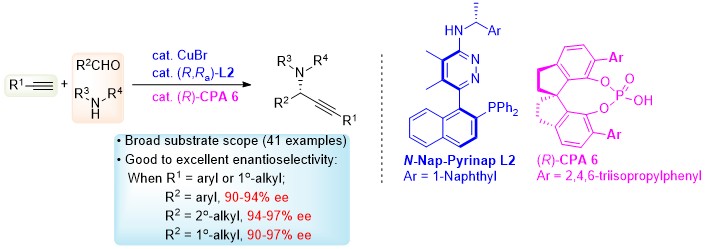
Due to their importance in natural products, organic synthesis, life science, and pharmaceuticals, reliable methods for the synthesis of chiral amines are urgently required. Here in this contribution, the dual chiral catalytic method has been established for enantioselective reaction of terminal alkynes, aldehydes, and amines affording optically active propargylic amines with a satisfied enantioselectivity enjoying a broad substrate scope. These propargylic amines have been demonstrated as platform molecules for the syntheses of different types of chiral amines. By applying this protocol, the asymmetric total syntheses of two linear monoamine natural products, (S)-5 and (S)-11, which were isolated from marine cyanobacteria Microcoleus lyngbyaceus, have been realized. The envisioned chiral ion pair, which has boosted the enantioselectivity greatly, and other key intermediates have been successfully characterized by MS studies.