NEWS

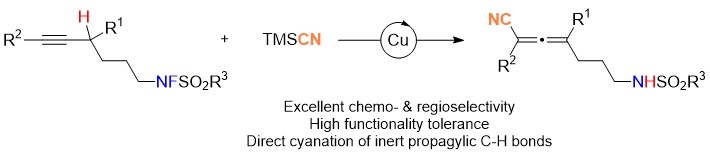
Warm congratulation on Dongjie Zhang's (张东杰) achievement on Cu-catalyzed propargylic C-H functionalization for allene syntheses.
Allenenitriles bearing different synthetically versatile functional groups have been prepared smoothly from 5-alkynyl fluorosulfonamides in decent yields with an excellent chemo- and regio-selectivity under redox neutral conditions. The resulting allenenitriles can be readily converted to useful functionalized heterocycles. Based on mechanistic study, it is confirmed that this is the first example of radical-based non-activated propargylic C-H functionalization for allene syntheses.