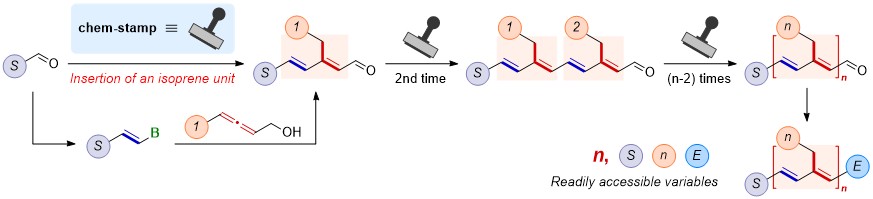
Many terpenoids with isoprene unit(s) demonstrating critical biological activities have been isolated and characterized. In this work, we have developed a robust chem-stamp strategy for the construction of the key isoprene unit, which consists of two steps: one-carbon extension of aldehydes to the alkenyl boronates via boron-Wittig reaction and the rhodium-catalyzed reaction of alkenyl boronates with 2,3-allenols yielding enals. This chem-stamp could easily be applied repeatedly and separately, empowering the modular concise syntheses of many natural and pharmaceutically active terpenoids including retinal, β-carotene, vitamin A, tretinoin, fenretinide, acitretin, ALRT1550, nigerapyrone C, peretinoin, and lycopene. Due to the diversified availability of the starting materials, aldehydes and 2,3-allenols, creation of new non-natural terpenoids has been realized from four dimensions: the number of isoprene units, side chain, and two terminal groups.