NEWS

Warm congratulation on Haibo Xu's (徐海波) achievement of Pd-catalyzed [6+2] double allene annulation!
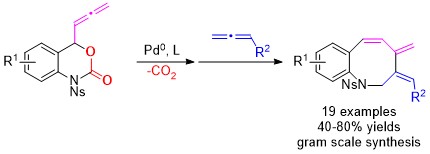
An efficient double allene protocol for the formation of benzazocines has been developed. The reaction constitutes a highly regioselective palladium-catalyzed formal [6+2] annulation of allenyl benzoxazinanones with terminal allenes forming the challenging 8-membered cycles. Decent yields and excellent regioselectivity have been observed under mild conditions with a remarkable Z -stereoselectivity for the exo-cyclic C=C bonds. The synthetic potentials of benzazocine products have been demonstrated. Link