NEWS

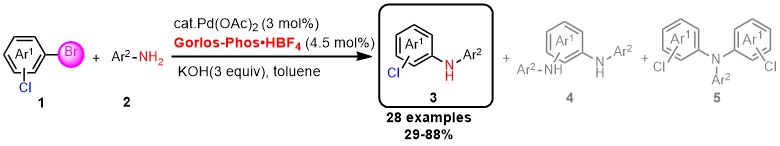
Warm congratulation on Xinyu Duan's (段鑫宇) achievement of Pd/Gorlos-Phos catalyzed amination reaction.
With the readily available Gorlos-Phos as a ligand, halogenated diarylamines were prepared efficiently from Pd-catalyzed monoamination of bromophenyl chlorides and primary arylamines, tolerating functional groups such as aldehyde, ketone, nitrile, fluorine, sulphonyl and trifluoromethyl groups. The formation of the amination of the C-Cl bond and diamination products were not observed.